SERVICES
A Global Reference Laboratories Pvt. Ltd. is dedicated to render its service in the following sectors:
- Stability studies
- Validation protocol for new products
- Analytical method validation.
- Technical training on good manufacturing practices/internal audits as per the need of drug authorities and the manufacturing units,institutions.
- Chemical & microbiological analysis of pharmaceuticals products,raw & packing materials, water & waste water, food & beverages and designing of packing materials and promotional items.
- Environmental studies.
- Studies on identifying substandard & counterfeit drugs in the market by strict surveillance in co-0peration with drug authorizes & consumer forum.
Agilent-HPLC 1260
The Agilent 1260 Infinity Quaternary LC offers the most flexibility for solvent
selection and automation in HPLC me
thod development, research and all
HPLC applications requiring continuous
access to a wide range of solvent
choices. The availability to rapidly switch between methods using different
solvents and the capability of using binary, ternary or quaternary solvent
gradients make the Agilent 1260 Infinity Quaternary LC the most flexible
system on the market.

UV Carry 60
The Agilent Cary 60 UV-Vis Spectrophotometer is efficient, accurate and flexible, and is designed to meet your immediate and future challenges. With remote sampling options, proven performance and low cost of ownership, you can be sure that the Agilent Cary 60 UV-Vis will give you answers you can trust.

Labindia Dissolution:
Labindia Dissolution Tester: DS 14000 (Basic) is uniquely designed to support USP 1, 2, 5 & 6 and is compliant with USP, BP, IP specifications. The 12+2 (6+1 & 6+1) vessel configuration comes with Moulded Water Bath which enables comparative studies

Fume Hood Veego:
A fume hood is typically a large piece of equipment enclosing five sides of a work area, the bottom of which is most commonly located at a standing work height.
Two main types exist, ducted and recirculating (ductless). The principle is the same for both types: air is drawn in from the front (open) side of the cabinet, and either expelled outside the building or made safe through filtration and fed back into the room. This is used to:
protect the user from inhaling toxic gases (fume hoods, biosafety cabinets, glove boxes)
protect the product or experiment (biosafety cabinets, glove boxes)
protect the environment (recirculating fume hoods, certain biosafety cabinets, and any other type when fitted with appropriate filters in the exhaust airstream)
Secondary functions of these devices may include explosion protection, spill containment, and other functions necessary to the work being done within the device.
Fume hoods are generally set back against the walls and are often fitted with infills above, to cover up the exhaust ductwork. Because of their recessed shape they are generally poorly illuminated by general room lighting, so many have internal lights with vapor-proof covers. The front is a sash window, usually in glass, able to move up and down on a counterbalance mechanism. On educational versions, the sides and sometimes the back of the unit are also glass, so that several pupils can look into a fume hood at once. Low air flow alarm control panels are common, see below.
Fume hoods are generally available in 5 different widths; 1000 mm, 1200 mm, 1500 mm, 1800 mm and 2000 mm.[1] The depth varies between 700 mm and 900 mm, and the height between 1900 mm and 2700 mm. These designs can accommodate from one to three operators.
For exceptionally hazardous materials, an enclosed glovebox may be used, which completely isolates the operator from all direct physical contact with the work material and tools. The enclosure may also be maintained at negative air pressure to ensure that nothing can escape via minute air leaks.

Flame Photometer
The compounds of the alkali and alkaline earth metals (Group II) dissociate into atoms when introduced into the flame. Some of these atoms further get excited to even higher levels. But these atoms are not stable at higher levels.
Hence, these atoms emit radiations when returning back to the ground state. These radiations generally lie in the visible region of the spectrum. Each of the alkali and alkaline earth metals has a specific wavelength.

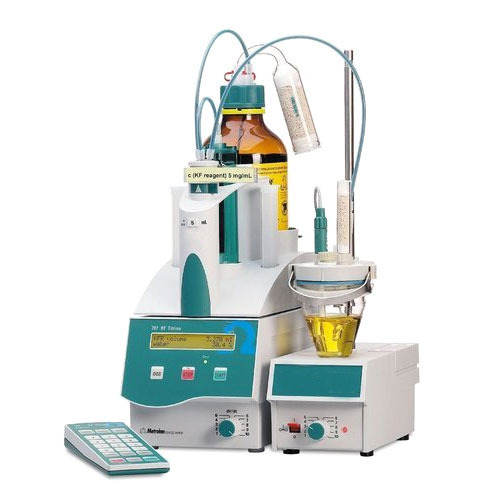
Karl Fischer
The Karl Fischer Titration is a titration method for measuring water content in basically all types of substances. It was invented in 1935 by the German chemist Karl Fischer.
The Karl Fischer Titration is based on an iodine / iodid reaction: The water reacts with iodine. The endpoint of the titration is reached when all the water is consumed
SO2 + CH3OH + B ↔ CH3SO3-+ HB+ CH3SO3-+ H2O + I2 + 2B → CH3SO4-+ 2HB++ 2I-
The process uses an organic base (B), sulphur dioxide, iodine and an alcohol. The original Karl Fischer method used pyridine as organic base and methanol as alcohol. Since then the reagents have been improved. Nowadays Karl Fischer reagents are available which are less toxic (no more pyridine but imidazole as organic base, ethanol instead of methanol) and which provide a faster reaction.
Polarimeter
Polarimetry measures the rotation of polarized light as it passes through an optically active fluid. The measured rotation can be used to calculate the value of solution concentrations; especially substances such as sugars, peptides and volatile oils. A polarimeter consists of a polarized light source, an analyzer, a graduated circle to measure the rotation angle, and sample tubes.
The polarized light passes through the sample tube and exhibits angular rotation to the left (-) or right ( ). On the side opposite the polarizer is the analyzer. Using optics, visual fields are manually adjusted by the user to measure the optical rotation angle.
Polarimeters offer high accuracies where precision is critical in determining the concentration of samples. offers manual polarimeters where you look through a viewing scope to read values on a vernier scale, and semiautomatic polarimeters that have a digital display. Polarimeters can measure in angle of rotation (¡), International Sugar Scale (°Z), or both.

IR Spectroscopy Machine
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) involves the interaction of infrared radiation with matter. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic techniques, it can be used to identify and study chemicals. Samples may be solid, liquid, or gas. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) to produce an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance (or transmittance) on the vertical axis vs. frequency or wavelength on the horizontal axis. Typical units of frequency used in IR spectra are reciprocal centimeters (sometimes called wave numbers), with the symbol cm−1. Units of IR wavelength are commonly given in micrometers (formerly called “microns”), symbol μm, which are related to wave numbers in a reciprocal way. A common laboratory instrument that uses this technique is a Fourier transform infrared (FTIR) spectrometer.

Tablet Disintegrator Machine
In pharmaceutical industry, disintegration is an essential test performed for testing disintegration capability of tablets and capsules etc as per pharmacopoeial standards like USP, BP and IP etc. Each pharmacopoeia standard has its own set of standards and specifies disintegration tests of its own.
The aim of this test is to monitor quality of different dosage forms and their performance through evaluating the time a formulation (tablet or capsule) takes to completely disintegrate. If disintegration time is found too high, it is considered that the tablet is highly compressed or the capsule shell gelatin is not adhering to pharmacopoeial quality. This test is also done to check consistency and uniformity of batches; if any kind of inconsistency is found and the samples fail to provide adequate results, appropriate actions can be taken on the basis of these results.

Lab RO System
Reverse osmosis (RO) is a water purification technology that uses a semipermeable membrane to remove ions, molecules and larger particles from drinking water. In reverse osmosis, an applied pressure is used to overcome osmotic pressure, a colligative property, that is driven by chemical potential differences of the solvent, a thermodynamic parameter. Reverse osmosis can remove many types of dissolved and suspended species from water, including bacteria, and is used in both industrial processes and the production of potable water. The result is that the solute is retained on the pressurized side of the membrane and the pure solvent is allowed to pass to the other side. To be “selective”, this membrane should not allow large molecules or ions through the pores (holes), but should allow smaller components of the solution (such as solvent molecules) to pass freely.[1]
In the normal osmosis process, the solvent naturally moves from an area of low solute concentration (high water potential), through a membrane, to an area of high solute concentration (low water potential). The driving force for the movement of the solvent is the reduction in the free energy of the system when the difference in solvent concentration on either side of a membrane is reduced, generating osmotic pressure due to the solvent moving into the more concentrated solution. Applying an external pressure to reverse the natural flow of pure solvent, thus, is reverse osmosis. The process is similar to other membrane technology applications. However, key differences are found between reverse osmosis and filtration. The predominant removal mechanism in membrane filtration is straining, or size exclusion, so the process can theoretically achieve perfect efficiency regardless of parameters such as the solution’s pressure and concentration. Reverse osmosis also involves diffusion, making the process dependent on pressure, flow rate, and other conditions.[2] Reverse osmosis is most commonly known for its use in drinking water purification from seawater, removing the salt and other effluent materials from the water molecules.

Lab Refrigerator Remi
New range of refrigerators from REMI are designed for storage applications in pharmaceutical, microbiology, biotechnology, chemical, clinical diagnostic, hospitals & research institutions. The unit is supplied with a glass door for convenient sample viewing, forced air circulation system ensures uniform temperature throughout the chambers. Digital controller is provided for display of internal temperature along with alarm systems.
Salient Features
Plastic inner chamber ; Outer body pre painted G.I.
Frost Free / Static Cooling. Energy efficient
Uniform cooling by forced air circulation
Adjustable Plastic coated wire mesh shelves
Clear product visibility with dual glass

